Structure crystal draw nacl cscl
Table of Contents
Table of Contents
If you’ve ever wondered how to draw the crystal structure of NaCl (sodium chloride), then you’ve come to the right place. Whether you’re a chemistry student or just someone who is interested in the topic, this guide will provide you with a step-by-step process to draw the crystal structure of NaCl.
When it comes to drawing crystal structures, there are often pain points that people encounter. For example, it can be difficult to visualize the three-dimensional structure of a crystal or to understand how the atoms are arranged in the unit cell. However, with the right approach and some practice, drawing a crystal structure can become much easier.
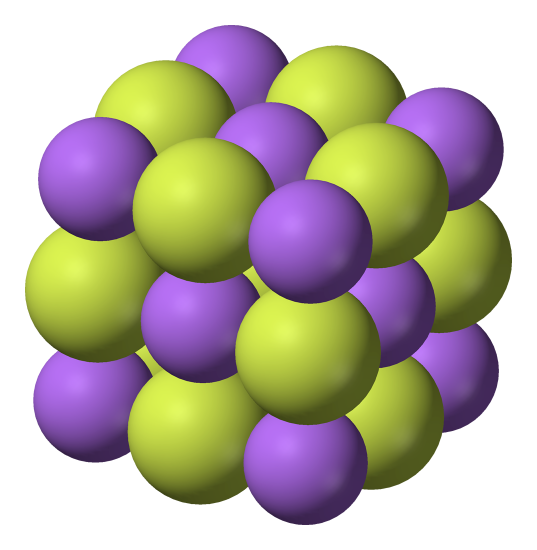
The first step to drawing the crystal structure of NaCl is to understand its basic structure. NaCl is made up of two ions, sodium (Na+) and chloride (Cl-), arranged in a face-centered cubic (fcc) lattice. This means that each ion is surrounded by six other ions, forming a cube-like structure.
To draw the crystal structure of NaCl, you’ll need to start with the unit cell, which is the basic repeating unit of the crystal. The unit cell for NaCl is a cube with an edge length of a. In the center of the cube is a sodium ion, and at each corner of the cube is a chloride ion.
My Experience with Drawing the Crystal Structure of NaCl
When I first learned how to draw crystal structures, I found it to be quite challenging. However, with practice and the right resources, I was able to improve my skills and gain a better understanding of these structures. Drawing the crystal structure of NaCl is no exception, but with the steps outlined above, you can make the process much easier.
The Step-by-Step Process of Drawing the Crystal Structure of NaCl
To draw the crystal structure of NaCl, you can follow these steps:
Step 1: Draw the Unit Cell
Start by drawing a cube with an edge length of a. This will be the unit cell for NaCl. In the center of the cube, draw a small circle to represent the sodium ion. At each corner of the cube, draw a smaller circle to represent a chloride ion.
 ### Step 2: Identify the Ions in the Unit Cell
### Step 2: Identify the Ions in the Unit Cell
Next, you’ll need to identify the sodium and chloride ions in the unit cell. The sodium ion is located in the center of the cube, while the chloride ions are at each corner.
Step 3: Connect the Ions
Now, connect the sodium ion to each of the chloride ions that are adjacent to it. You should have six connections in total.
Example:
 ### Step 4: Repeat the Unit Cell
### Step 4: Repeat the Unit Cell
Finally, you’ll need to repeat the unit cell to create the entire crystal structure. To do this, simply stack the unit cells on top of one another in a fcc lattice, making sure that the sodium ions are aligned with the chloride ions of adjacent unit cells.
Tips for Drawing Crystal Structures
When drawing crystal structures, there are a few tips that can be helpful:
- Start by drawing the unit cell and identifying the ions.
- Connect the ions using simple lines or curves.
- Repeat the unit cell to create the entire crystal structure.
- Use different colors to differentiate between the different types of ions.
Question and Answer
Q: What is NaCl’s crystal structure?
A: NaCl has a face-centered cubic (fcc) crystal structure.
Q: What is the unit cell for NaCl?
A: The unit cell for NaCl is a cube with an edge length of a.
Q: How are the sodium and chloride ions arranged in NaCl?
A: The sodium and chloride ions in NaCl are arranged in a fcc lattice, with each sodium ion surrounded by six chloride ions and vice versa.
Q: How do you draw the crystal structure of NaCl?
A: To draw the crystal structure of NaCl, start by drawing the unit cell and identifying the ions. Then, connect the ions using simple lines or curves, and repeat the unit cell to create the entire crystal structure.
Conclusion of How to Draw Crystal Structure of NaCl
Drawing the crystal structure of NaCl can be challenging, but with the right approach and some practice, it can become much easier. By following the steps outlined above, you can learn how to draw NaCl’s crystal structure and gain a deeper understanding of this important chemical compound.
Gallery
The Edge Length Of The Rock Salt Type Unit Cell Is Class 11 Chemistry CBSE
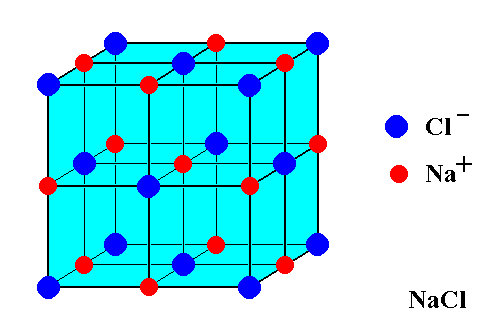
Photo Credit by: bing.com / nacl reticolare structure sodio cloruro lattice chimicamo chemistry salzkristalle atoms struttura aufbau ions wieso rechteckig meine kristallgitter formula
Draw The Crystal Structure Of NaCl And CsCl? - Brainly.in

Photo Credit by: bing.com / structure crystal draw nacl cscl
How To Draw Crystal Structure Of NaCl ,IIT, - YouTube

Photo Credit by: bing.com / structure nacl draw crystal
How Many Chloride Ions Are There Around Sodium Ions Class 12 Chemistry CBSE
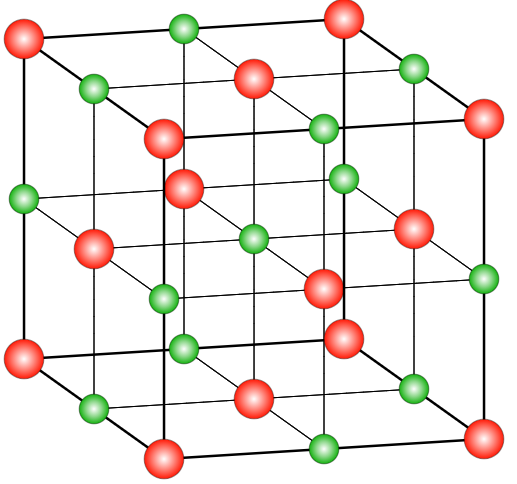
Photo Credit by: bing.com / chloride ionic ions enthalpy periodic spheres whereas pngguru
Chapter 4.1: Ionic Bonding - Chemistry LibreTexts

Photo Credit by: bing.com / ionic bonding sodium sodio fluoruro nacl lattice libretexts fluoride chloride ionenbindung ions barniz carefully molecular pngegg





